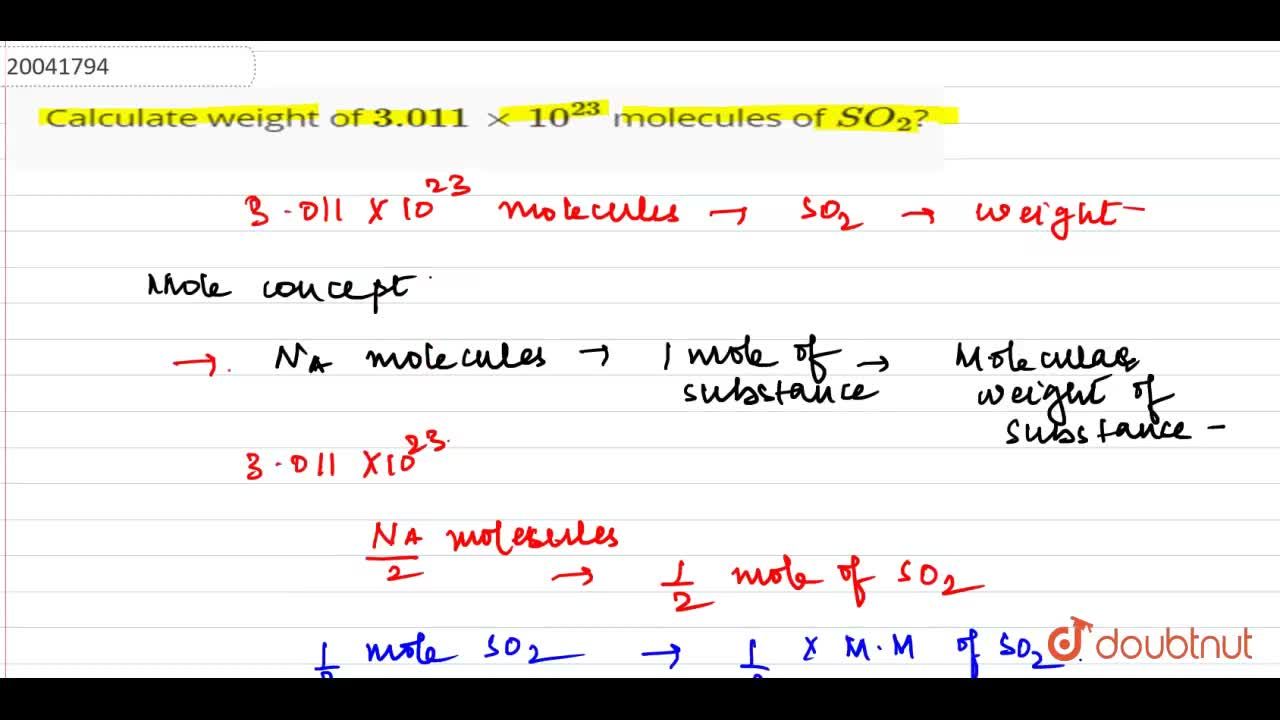
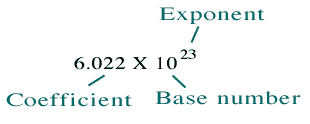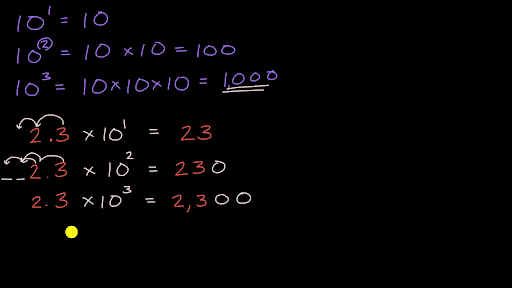Multiply 0.2 x 6.022 x 10^23 Need step by step explanation no direct answer or else get reported and ID - Brainly.in
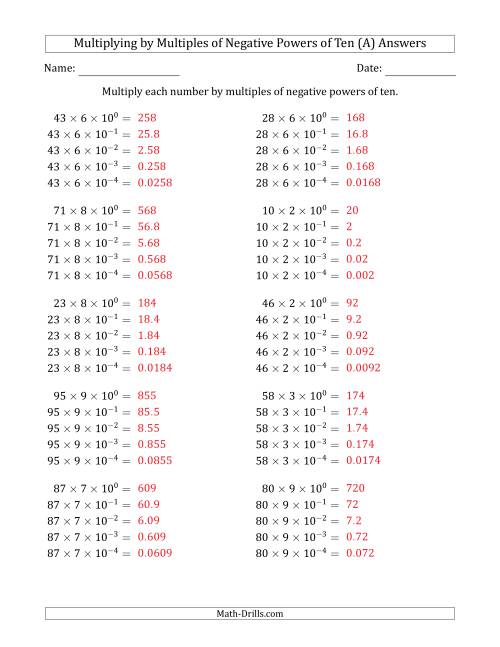
Learning to Multiply Numbers (Range 10 to 99) by Multiples of Negative Powers of Ten in Exponent Form (A)
57.suppose a gas sample in all have 6*1023molecules. each 1/3 of the molecules have r.m.s. speed 104cm/sec, 2*104cm/sec, 3*104cm/sec. calculate the r.m.s. speed of gas molecules in sample

SF 03 - Multiplying by Powers of 10 | SF 03 - Multiplying by Powers of 10 The 4th in the Standard Form Series... Teaches how to multiply whole numbers and decimals
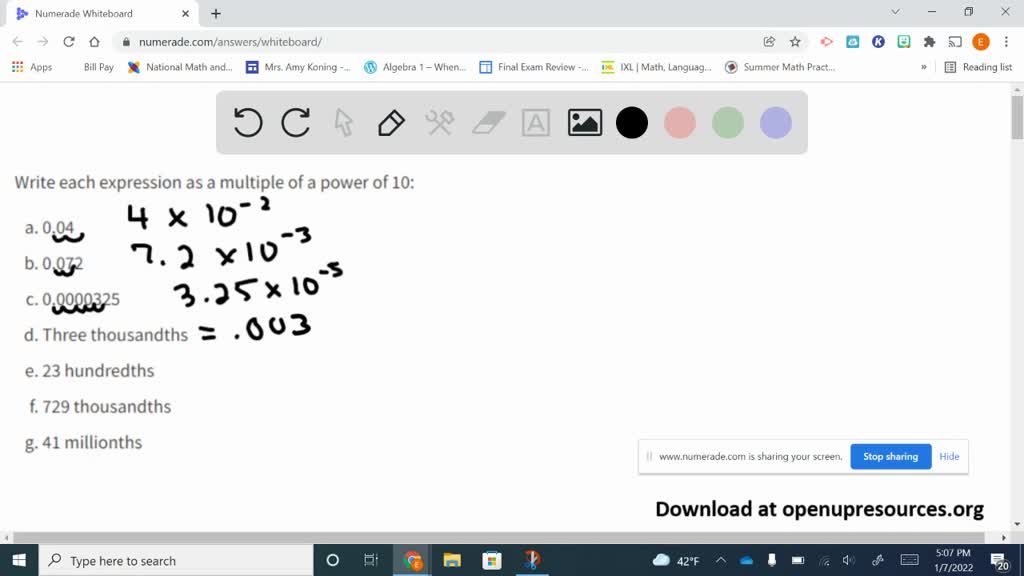
SOLVED:Write each expression as a multiple of a power of 10: a. 0.04 b. 0.072 c. 0.0000325 d. Three thousandths e. 23 hundredths f. 729 thousandths g. 41 millionths

SCIENTIFIC NOTATION A value written as the product of two numbers: a coefficient and 10 raised to a power. Ex: 602,000,000,000,000,000,000,000 is ppt download